Use of thermogravimetry to study CO2 sorption
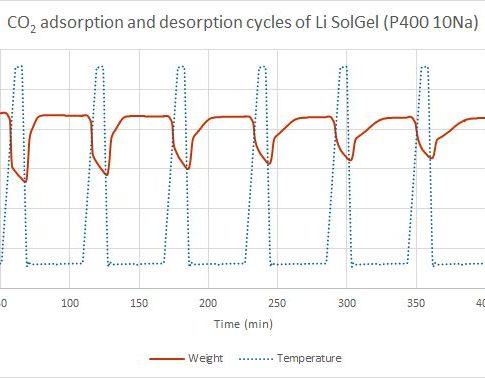
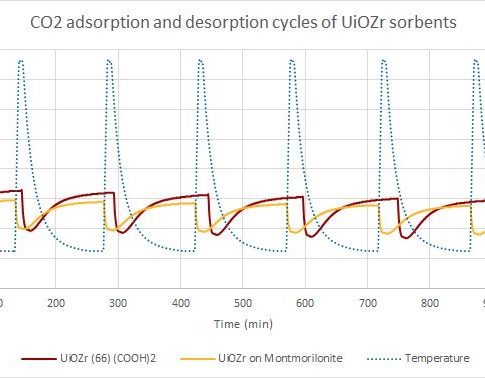
Thermogravimetry is considered as one of methods to characterize the ability of porous materials to adsorb carbon dioxide. The method is based on the material mass changes as a function of temperature.
After the sample is weighed into a porcelain crucible, the desorption phase is started.
In this phase, in an inert gas atmosphere, the sample is heated to the desired temperature, where different volatile compounds, such as water vapor and other gases, are released from the sample. In case of high temperature sorption, desorption takes place at 750 ° C, but in low – 150 ° C. When the temperature is reached, the next phase can take place.
The adsorption phase takes place in a carbon dioxide atmosphere. During this phase, the mass of the sample increases as it adsorbs and absorbs carbon dioxide from the environment during gradual decrease of temperature. At a given time and given temperature the sample has taken up maximum amount of carbon dioxide. In case of high-temperature sorption, this occurs at about 650 ° C, and at low temperatures – at 25 to 75 ° C.
The adsorption and desorption temperatures may be different for each material.
Adsorption and desorption cycles are repeated. Thus, the ability of a material CO2 re-absorption and how it decreases over time is considered.